The Fetal Cell Count™ Kit (CE/IVD) – A complete assay for the routine diagnosis of Foetomaternal Hemorrhage using Anti-CA and Anti-HbF.
Key Features
- Unique quantification of Fetomaternal Hemorrhage by flow cytometry
- Complete assay for routine diagnosis of Fetomaternal Hemorrhage
- Detection as low as 0.02% fetal cells in maternal blood
- Detection of fetal haemoglobin (HbF) and Carbonic Anhydrase (CA)
- Simple and easy handling procedure
- IVD/CE registered
- Determination of Fetomaternal Hemorrhage
- Pregnancy with suspected RhD incompatibilities
- Abdominal trauma
- Recognize fetal cells in maternal Thalaessemia circulation
Product Information
 Product Code: IQP-363
Product Code: IQP-363
Product Name: Fetal Cell Count™ Kit
Size: 25 Tests
Regulatory Status: CE/IVD
View the Product Information Sheet Here
Principle of the Fetal Cell Count™ Kit
The Fetal Cell Count™ Kit assay is a flow cytometry product. The assay is based on a patented combination of two antibodies. One is directed against HbF while the second is specific for carbonic anhydrase (CA), an enzyme present in adult RBCs and, at very low detectable level, in late pregnancy stage.
- Results within 90 minutes
- Precise and accurate detection of fRBCs
- Intracellular detection of HbF and CA
- Distinguishes between fRBCs and maternal F-cells and RBCs, it eliminates subjective interpretations an empiric signal cutoffs
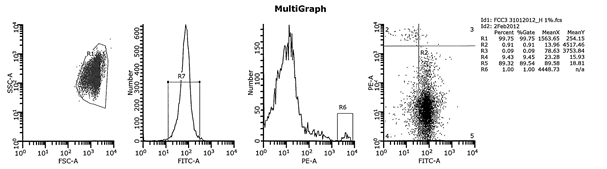
Fig 1. Cytograms illustrating typical data obtained with the Fetal Cell Count Kit A1; fetal RBCs / A2; F-cells / A4; maternal RBCs.
Background
Detection and quantification of fetal red blood cells (fRBCs) in maternal blood samples is essential for obstetrical management. Measurement of fRBCs is critical as the extent of Fetomaternal Hemorrhage (FMH), the transplacental passage of fRBCs into the maternal circulation, has consequences for further treatment of mother and child. Frequency and size of FMH is directly influenced by complications in abdominal trauma, suspected placental injury or after a caesarean section. Severe FMH may lead to intra-uterine death. In case of antigen incompatibility between mother and child FMH may result in respiratory problems or anaemia, like Haemolytic Disease of the Newborn.
Adult RBC’s contains a population of HbF (F-cells), which can be differ between 0 and 14%. The F-cells can give a positive result in the Kleihauer-Betke acid-elution test or single HbF flow cytometry test. These F-cells may be the result of physiological variations during pregnancy or traits such as thalassemia, sickle cell anaemia or hereditary persistence of fetal haemoglobin.
The detection (and thus enumeration) of fRBCs is used to calculate the extent of FMH, either in case of trauma with suspected placental injury or in the situation of a RhD incompatibility between the fetus and the mother. The amount of fRBCs is a measure for the prevention of hemolytic disease of the newborn using (prophylactic) anti-D therapy.
Further Information
The Foetal Cell Count™ Kit developed by IQ Products is distributed in the UK by Caltag Medsystems. For more information about this product, or any other reagents from IQ Products, please contact us and we will be happy to assist you.
For references, FAQs and more, please visit: http://www.iqproducts.nl/products/perinatal/fetal-cell-count-kit-diagnosis-of-fetomaternal-hemorrhage
Also Available
FETALtrol – Tri-level stabilised blood controls containing known concentrations of human foetal erythrocytes in human adult blood.